Quick Exam Notes Science for Upper Secondary are specially compiled to help students prepare for important tests and examinations.
Clear Presentation Format
Notes are presented in point form for ease of understanding and systematic learning. Students will be able to review important concepts and examples quickly.
Useful Illustrations
A variety of diagrams, graphs and tables are included. Students will be able to understand concepts and processes easily through these helpful visual aids.
Contents
CHAPTER 1: Experimental Chemistry
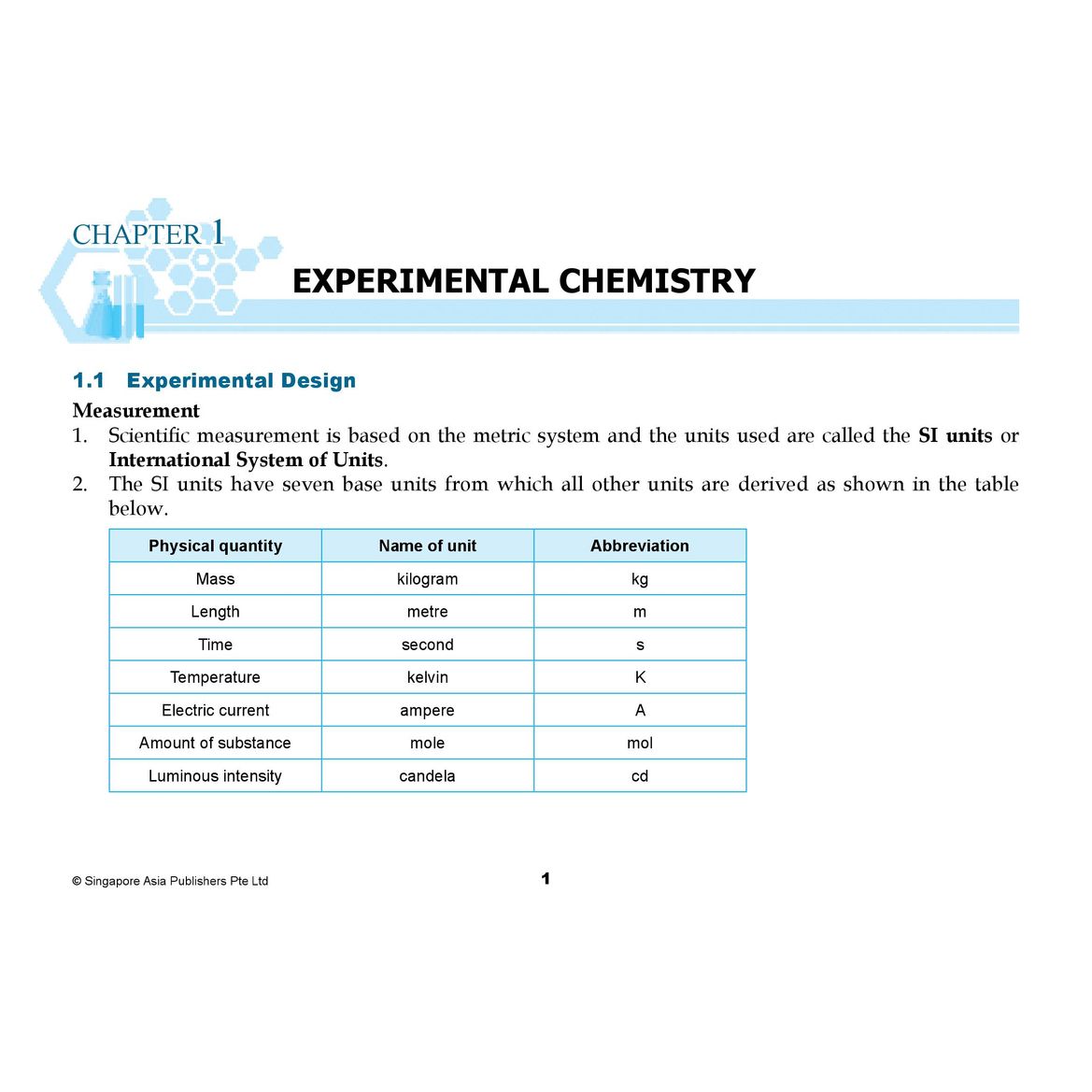
- 1.1 Experimental Design
- 1.2 Methods of Purification and Analysis
- 1.3 Pure Substances
- 1.4 Identification of Ions and Gases
CHAPTER 2: The Particulate Nature of Matter
CHAPTER 3: Formulae, Stoichiometry and the Mole Concept
- 3.1 Writing Chemical Formulae
- 3.2 Chemical Equations
- 3.3 Ionic Equations
- 3.4 Relative Atomic Mass (Ar)
- 3.5 Relative Molecular Mass (Mr)
- 3.6 The Mole
- 3.7 Molar Mass
- 3.8 Percentage Composition
- 3.9 Empirical Formula and Molecular Formula
- 3.10 Molar Volume
- 3.11 Limiting Reactants
- 3.12 Molar Solution
- 3.13 Percentage Yield
- 3.14 Percentage Purity
CHAPTER 4: Electrolysis
- 4.1 Electrolysis
- 4.2 Electrolysis of Molten Electrolytes
- 4.3 Electrolysis of Aqueous Electrolytes
- 4.4 Industrial Application of Electrolysis
- 4.5 Simple Electric Cells
CHAPTER 5: Energy from Chemicals
- 5.1 Enthalpy Changes
- 5.2 Exothermic Reactions
- 5.3 Endothermic Reactions
- 5.4 Bond Forming and Bond Breaking
- 5.5 Fuels
CHAPTER 6: Chemical Reactions
- 6.1 Speed of Reaction
- 6.2 Factors Affecting Speed of Reaction
- 6.3 Investigating the Effect of a Given Variable on the Speed of a Reaction
- 6.4 Redox Reactions
- 6.5 Testing for Oxidising and Reducing Agents
CHAPTER 7: Acids, Bases and Salts
- 7.1 Acids
- 7.2 Bases
- 7.3 pH Scale
- 7.4 Types of Oxides
- 7.5 Preparation of Salts
- 7.6 Manufacture and Uses of Ammonia
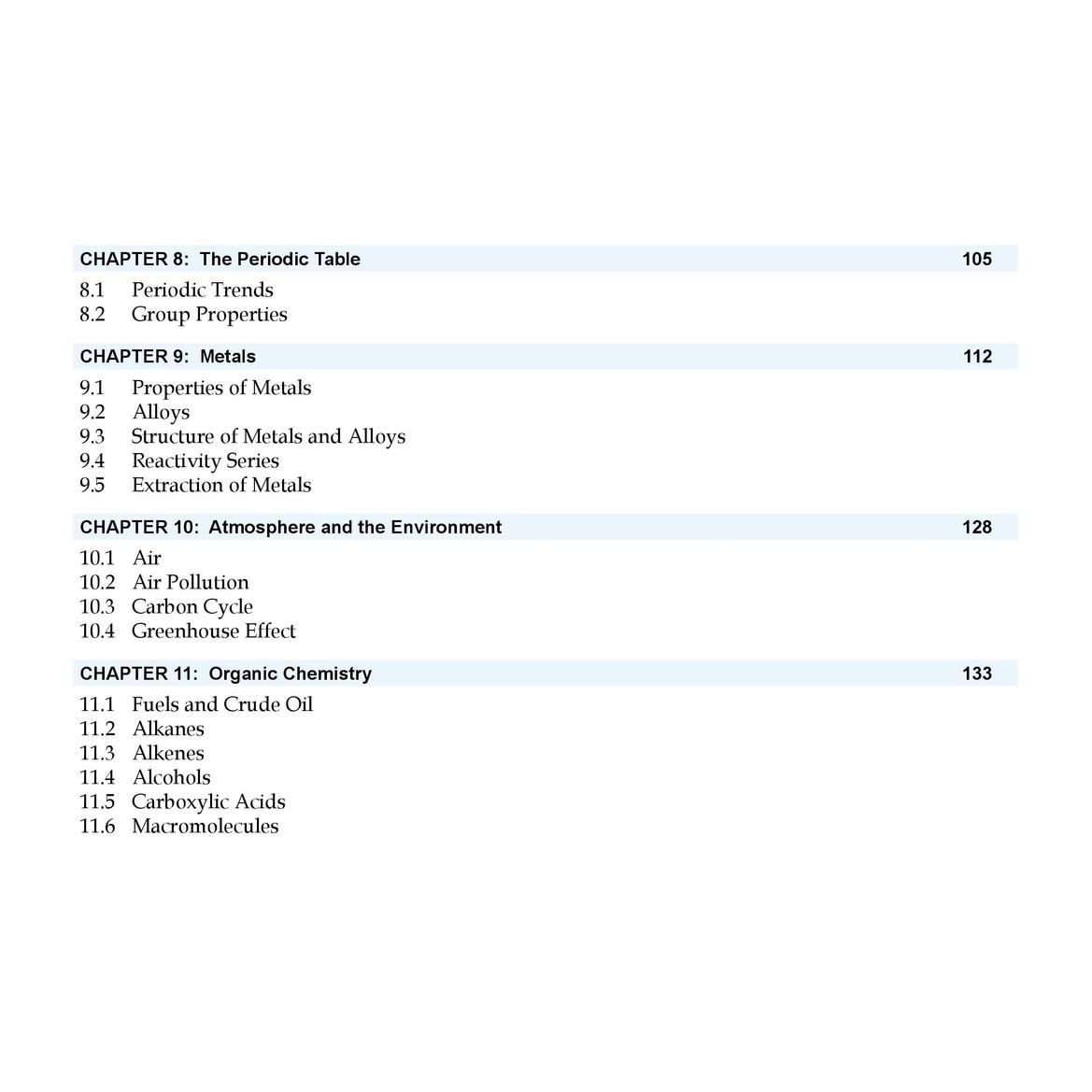
CHAPTER 8: The Periodic Table
- 8.1 Periodic Trends
- 8.2 Group Properties
CHAPTER 9: Metals
- 9.1 Properties of Metals
- 9.2 Alloys
- 9.3 Structure of Metals and Alloys
- 9.4 Reactivity Series
- 9.5 Extraction of Metals
CHAPTER 10: Atmosphere and the Environment
- 10.1 Air
- 10.2 Air Pollution
- 10.3 Carbon Cycle
- 10.4 Greenhouse Effect
CHAPTER 11: Organic Chemistry
- 11.1 Fuels and Crude Oil
- 11.2 Alkanes
- 11.3 Alkenes
- 11.4 Alcohols
- 11.5 Carboxylic Acids
- 11.6 Macromolecules
ISBN : 9789814672962
Number of Pages : 160
Author : A. B. Terence